Hypochlorous Acid
- HOCl (Electrolyzed Water)
What is Hypochlorous acid?
Hypochlorous acid (also known as Electrolyzed Water) is nature’s
oldest disinfectant, and it happens to be walking around in about
7.5 billion people right now. As in inside all humans, this very
moment.
Why? It’s the substance your white blood cells produce to fight
off infections. It’s also the active ingredient in electrolyzed
water, which is an industrial technology used for green cleaning
and sanitizing. Electrolyzed water is made when electricity is
used to change the chemical structure of salt, water & vinegar
into a green cleaner as effective as bleach, but with no harmful
chemicals, fumes or residues.
How is hypochlorous acid made?
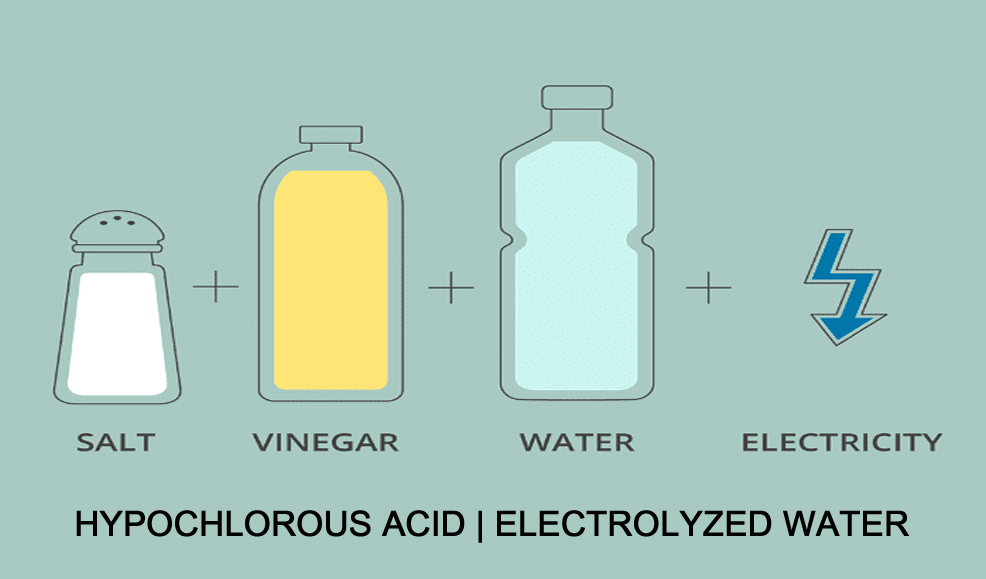
To make hypochlorous acid is pretty simple chemistry. Start with
precisely the correct proportions of three ingredients: salt,
water and vinegar. You may remember that a salt molecule is made
up of the elements sodium and chloride (NaCl) and a water molecule
is made up of hydrogen and oxygen (H2O). When an electrical
current is applied to the solution, the molecules break apart and
the elements form two new molecules:
Hypochlorous acid (HOCl)
Nature’s super powerful disinfectant, and also the ingredient that
gives bleach its anti-microbial power. When the pH of the solution
is lowered to the correct level, HOCl is created, exactly the same
substance that is your immune system’s germ fighter. So when you
take your child to that indoor play area/petrie dish – your white
blood cells get to work creating hypochlorous acid.
Healthcare & medical uses of
hypochlorous acid
Hypochlorous acid is so gentle that it has several uses in the
healthcare and medical space. It’s FDA approved for use in wound
healing, wound care and eye care products and is also common in
veterinary care products. It’s even used to eradicate biofilm.
There has been extensive research on the gentleness and efficacy
of HOCl when it comes to killing bacteria. It is so trusted and
effective, that hospitals use it as a disinfectant in both the US
and Japan.
Hypochlorous acid is naturally produced by white blood cells in
all mammals for healing and protection. It plays an important role
in the immune system killing pathogens through oxidation and
chlorination.
Coronavirus Disease (COVID-19)
Coronavirus Disease 2019 (COVID-19) is a novel virus. It causes
severe acute respiratory syndrome.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is
the agent responsible for a surface-to-surface communicable
disease.
On contact with the virus, the hypochlorous acid (HOCl) changes
the protective protein coat, which loses its structure and
aggregates, forming clumps of proteins with other viruses.
Currently, the US Environmental Protection Agency has recommended
hypochlorous acid (HOCl) disinfectant against COVID-19.
The mechanism of disinfection involves the destroying of the cell
wall of microbes or viruses, allowing the disinfectant to destroy
or inactivate them.
Hypochlorous Acid listed at EPA's
List N
Environmental Protection Agency (EPA) confirms that Hypochlorous
Acid (HOCl) eliminates 99.99% of COVID-19 in just 20 seconds,
faster than any disinfectant included on the EPA List N.
Hypochlorous Acid (HOCl) is the
disinfectant approved for use by our robots
After conducting laboratory and operation tests with various
disinfectants considered for the combat of COVID-19, our company
opted to homologate hypochlorous acid as the disinfectant to be
used by our robots.
Spray disinfection of hypochlorous acid HOCl) eliminates 99.99% of
COVID-19 in just 20 seconds, without harming people's health, and
is faster than any other disinfectant included on the EPA List N.
Safe on Eyes and Skin -
Non-Toxic, Non-Hazardous
Hypochlorous acid does not cause irritation to eyes and skin. Even
it were ingested it causes no harm. Because it is so safe, it is
the ideal sanitizer for direct food sanitation and food contact
surfaces. It is also ideal in healthcare where it is used for
wound cleansing, eye drops, and patient room disinfection
replacing toxic chemicals such as bleach and quaternary ammonium
(quats).
Sanitation chemicals distributed in concentrated form are toxic
and can be hazardous. Contact with skin or inhalation of fumes can
cause irritation. These risks do not exist with hypochlorous acid.
Electrolyzed water systems generate hypochlorous acid from just
table salt, water and electricity. No personal protective gear is
required.
Why is HOCl more efficient at
killing pathogens?
Hypochlorous Acid (HOCl) vs.
Sodium Hypochlorite (Chlorine Bleach)
- The Hypochlorite ion carries a negative electrical charge,
while hypochlorous acid carries no electrical charge.
- The hypochlorous acid moves quickly, able to oxidize the
bacteria in a matter of seconds, while the hypochlorite ion
might take up to a half hour to do the same.
- Germ surfaces carry a negative electrical charge which
results in a repulsion of the negatively charged hypochlorite
ion to the area of the germ surfaces, making hypochlorite ion
less effective at killing germs.
- The hypochlorous acid' slack of electrical charge allows it
to more efficiently penetrate the protective barriers
surrounding germs.
New Technology & Research
The use of chlorine for disinfection has been researched for over
100 years. It has been an undisputable fact that hypochlorous acid
offers far superior disinfecting properties than sodium
hypochlorite (chlorine bleach). One of the most well known
authorities for the use of chlorine as a disinfectant is White's
Handbook of Chlorination. This book is comprehensive in explaining
the chemistry and effectiveness of chlorine and alternative
disinfectants.
The challenge has been in engineering a system for producing a
free chlorine solution that is dominated by the molecule of
hyopchlorous acid (HOCl) rather than sodium hypochlorite (NaOCl-).
The development of electrolysis cells for generating electrolyzed
water became a huge innovative breakthrough in the 1970s. Since
then, improvements in electrolysis cells have been made that can
generate a solution of free chlorine that is near 99% hypochlorous
acid and that is stable.
One of the most recent improvements has been the development of
single cell technology to replace membrane cell technology
allowing for the production of just one stream of solution at a
near neutral pH. Prior technology used membranes and high
pressures that forced two streams to be generated, an unstable
anolyte of hypochlorous acid and an unstable catholyte of sodium
hydroxide. With the development of single cell technology, a
stable solution of just anolyte can be produced yielding a
solution of near 99% stable hypochlorous acid.
Over 30 years of research exisits for the use of hypochlorous acid
and new research is being published every year. Recent research
has focused on the use of hypochlorous acid for sanitzing food and
food processing facilities. Research has also been done on poultry
farms, water treatment and disinfection, and healthcare related
applications such as wound care and equipment sterilization.
Hypochlorous acid is also produced through a process called
electrolysis. Electrolysis is a technique that uses a direct
electric current (DC) to drive an otherwise non-spontaneous
chemical reaction. Specifically engineered elecrolysis cells can
generate a solution of free chlorine species by running
electricity through NaCl (table salt) and water. The oxidants
hypochlorous acid (HOCl) and hypochlorite (OCl-) are formed at the
anode. If the pH of the solution is weakly acidic to neutral, the
free chlorine solution will be dominated by hypochlorous acid.
Hypochlorous is a powerful oxidant and is 100 times more efficient
at killing microbial pathogens than sodium hypochlorite (aka.
chlorine bleach). |